Correct Answer

verified
Correct Answer
verified
Short Answer
Instructions: Determine the hybridization for the indicated atoms in each structure below.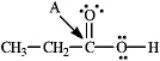
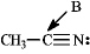 Refer to instructions.The hybridization of carbon atom A is _____.
Refer to instructions.The hybridization of carbon atom A is _____.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents the shape of a 2p atomic orbital of carbon? 
A) 1
B) 2
C) 3
D) 4
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw all the lone pairs (nonbonding valence electrons)on the structure of phosgene,a poisonous gas once used as a chemical warfare agent.
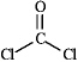
Correct Answer

verified
Correct Answer
verified
Essay
Instructions: Propose a structure for a molecule that meets the following description. Refer to instructions.Contains only two sp3 hybridized carbons and two sp hybridized carbons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true?
A) The carbon-carbon single bond of an alkane is weaker than the carbon-carbon triple bond of an alkyne.
B) The carbon-carbon triple bond of an alkyne is shorter than the carbon-carbon double bond of an alkene.
C) The carbon-carbon triple bond of an alkyne is exactly three times as strong as a carbon-carbon single bond of an alkane.
D) The carbon-carbon single bond of an alkane is longer than the carbon-carbon triple bond of an alkyne.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true according to molecular orbital (MO) theory?
A) Antibonding orbitals are higher in energy than the corresponding bonding orbital.
B) The head-on overlap of an s and a p atomic orbital can produce a s molecular orbital.
C) A p molecular orbital forms only from the combination of p atomic orbital wave functions.
D) The subtractive combination of atomic orbital wave functions produces a bonding molecular orbital.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Specify the hybridization of each carbon atom of limonene,a natural product present in citrus fruits,and thujone,which is derived from wormwood,a traditional component of the notorious liquor,Absinthe.
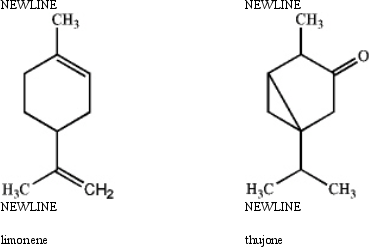
Correct Answer

verified
Correct Answer
verified
Short Answer
Instructions: Determine the hybridization for the indicated atoms in each structure below.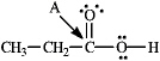
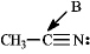 Refer to instructions.The hybridization of carbon atom B is _____.
Refer to instructions.The hybridization of carbon atom B is _____.
Correct Answer

verified
Correct Answer
verified
Essay
Draw all possible structures of CFnClm where n and m vary from 0 to 4.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 30 of 30
Related Exams